Key words:
Particle characterization, Nanoparticle Tracking Analysis (NTA), Brownian Motion Size, Electrophoretic Mobility, Zeta Potential, Single Particle Fluorescence
Summary:
- Introduction to the measurement principle of NTA
- From Brownian Motion to particle size distributions
- Multi-parameter technique: size, concentration, zeta potential and fluorescence
Introduction
Nanoparticle Tracking Analysis (NTA) is one of the few methods to visualize and measure nanoparticles in suspension in the range from 10 – 1000 nm based on the analysis of Brownian motion. Objects with two dimensions smaller than 100 nm are termed nanoparticles or ultrafine particles [1,2]. Fine particles are sized between 100 and 2500 nm. The size is the translational diffusion diameter of a sphere, the so called hydrodynamic diameter.
Nanoparticles with their fascinating physical properties improve e.g. fridges with antibacterial coatings or dyes and pigments in their appearance in sunlight. In quality control, analysis of size and concentration need to be determined from a day to day basis with emphasis on reliability and speed. Comprising analysis including size, concentration, surface charge (zeta potential) and fluorescence is needed by researchers to gain deep insights in processes of synthesis, reaction kinetics or specificity studies. NTA is a versatile technique capable of multiparameter measurement for all kinds of particles, saving time and sample amount. The applications of NTA range from quantification of size, concentration or zeta potential of inorganic particles such as silicon dioxide, polymers, calcites, phosphates, barium sulfate to carbon nanotubes or nanobubbles. In addition, all kinds of bionanoparticles such as protein aggregates, extracellular particles (such as exosomes or EVs), viruses or liposomes can be analyzed and discriminated by fluorescent labeling.
Measurement Principle
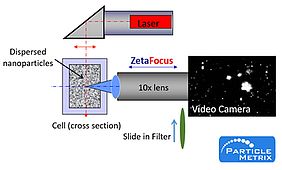
NTA determines the Brownian motion by analysis of a video sequence. Particles in the sample are visualized by the illumination with a laser beam. The scattered light of the particles is recorded with a light sensitive CCD or CMOS camera, which is arranged at a 90° angle to the irradiation plane. The 90° arrangement, also known as Ultra microscopy [3] allows detection and tracking of the Brownian motion of 10 to 1000 nm sized vesicles. Using a special algorithm, particles are detected and their path registered.
The size of each individually tracked particle is calculated, thus simultaneously allowing determination of their size distribution and concentration.
Even though the NTA technology is relatively new on the market, it originated almost 25 years ago [4]; the commercial implementation of this technique required the availability of fast computer systems that are able to cope with the computationally intensive video analysis in reasonable time frames. A detailed procedure on the practical procedure for NTA measurement is given in the ASTM standard for NTA measurements [5].
Physical principle
A brief introduction of the physical principle underlying NTA is as follows: When small particles are dispersed in a liquid the particles move randomly in all directions. The liquid is also termed continuous phase and can be water, or organic solvents such as 70% ethanol. The phenomenon of the random movement is termed diffusion and is expressed by the diffusion coefficient (D). In more detail, the undirected migration of given particles is caused by energy transfers from surrounding water molecules to the particle. The theory of the determination of the diffusion coefficient was developed from the famous physicist Albert Einstein [6]. In the absence of any concentration gradient within the dispersion and upon long-term observation, the distances small particles move in any direction should neutralize each other over time, leaving a total movement of almost zero. However, during given time intervals diffusing particles move within certain volume elements. In NTA the time t between two observation spots is quite short (~30 ms). The movement of the particles per time interval is recorded and quantified as the mean square displacement <x2>. Depending on the number of dimensions (one, two or all three dimensions) the observed mean square displacement, the diffusion coefficient, can be calculated as follows:
Via the Stokes-Einstein relationship the particle diameter d can be calculated as function of the diffusion coefficient D at a temperature T and a viscosity η of the liquid (κB Boltzmann’s constant)
In NTA, the particle fluctuation of a single particle is registered in two dimensions. After combining the Stokes-Einstein relationship and the two dimensional mean square displacement, the equation can be solved for the particle diameter d with:
By simultaneously tracking several particles their diameters can be determined in parallel. The lower limit of the working range, i.e. the smallest detectable particle size, depends on the scattered intensity of the particle, the efficiency of the magnifying optics and the sensitivity of the camera. Nanoparticles of silver and gold are strong scatterers due to the comparably large refractive indices of 2-4 and can be detected down to sizes of ~10 nm. Biological nanoparticles such as EVs have refractive indices of around 1.37-1.45 resulting in a limit of detection of 30-50 nm for NTA [7].
NTA allows the direct measurement of concentration as single particles in the illuminated volume are visualized. Thus, NTA is an absolute measurement technique allowing the determination of total surface or volumes of particles in a sample. For the measurement of concentration, the instrument is calibrated with size standards of known size and concentration. The visualization of the sample gives a unique impression on the quality of the sample, such as the presence of agglomerates. The working range of 105 and 109 particles per cm3 is very low compared to DLS, allowing NTA to analyze low concentrated samples. To record representative size distribution profiles it is recommended to analyze a range of 1,000–10,000 single particles. Temperature control, automatic focusing, and conductivity measurement is integrated.
The quality of an NTA result is influenced by particle contamination. In addition, high concentrations of stabilizing agents (e.g. surfactants) are critical as soon as they reach their critical micellar concentration (CMC). Contamination in the form of particles may derive from diluents (distilled water or buffer agents) or from chemicals used during preparation of samples. In general, content of particulate matter of chemicals after dissolving is not certified. Precipitates of phosphates, carbonates or silicates as well as dust can be removed filtration of the buffers, ideally with pore sizes below 50 nm. Degassing in ultrasonic bath is also helpful to remove air bubbles.
NTA is a multi-parameter technique: size, concentration, zeta potential and fluorescence
The main application when analysis of particles is the determination of size and concentration. However, to understand underlying processes or quantify and discriminate particles, zeta potential measurement and fluorescence detection are integrated for comprising particle characterization.
Size
Particle size of single particles is calculated from the Brownian motion analysis. The particle size distribution is created by accumulation of sizes of several 100 to 1000 individual particles.
Concentration
By knowing the measurement volume, the concentration is determined by counting all objects in the field of view. With normalization of the measurement volume, the particle concentration can be given in number of particles per cm3, as well as area and volume, marking out the NTA technique as an absolute measurement.
Zeta potential
The zeta potential reflects the surface charge of given particles, which is related to their stability by electrostatic forces. As charge of nanoparticle changes when surface has different coating, particles of same size but different surface coating can be differentiated. For example, conjugated and unconjugated gold nanoparticles show difference in zeta potential.
Fluorescence detection
The detection of fluorescence, for example vesicles tagged with specific fluorescence dye empowers NTA to specific detection for the quantification of different subpopulations. With 488 nm laser, the instrument detects vesicles, EVs or virus like particles (VLPs) tagged with typical dyes such as Alexa488, PKH67 and GFP.
Summary
NTA is particle characterization technique for practically all kinds of nanoparticles suspended in media. On the same sample, size, concentration, zeta potential and fluorescence on the same sample are measured.
The highlights of this technique are:
- Characterization of nanoparticles in solution
- Measurement at comparably low concentration levels (down to 105 particles per cm-3)
- Single particle analysis resulting in high resolution distributions
- Specificity with fluorescent detection
NTA is a fast single particle analysis tool to visualize and quantify size, concentration, surface charge (zeta potential) and fluorescence.
References
[1] ISO/TS 80004-2:2015(en) Nanotechnologies — Vocabulary — Part 2: Nano-objects
[2] ASTM E2456-06:2012, Standard Terminology Relating to Nanotechnology
[3] Zsigmondy, R., Colloids and the Ultra Microscope, J. Am. Chem. Soc., 1909, 31 (8), pp 951–952. doi: 10.1021/ja01938a017
[4] Qian, H., Sheets, M. P., Elson, E. L., Single particle tracking, Biophys J. 1991 Oct; 60(4): 910–921. doi: 10.1016/S0006-3495(91)82125-7
[5] ASTM E2834-12, Standard Guide for Measurement of Particle Size Distribution of Nanomaterials in Suspension by Nanoparticle Tracking Analysis (NTA), ASTM International, West Conshohocken, PA, 2012, www.astm.org
[6] Einstein, A., Annalen der Physik, 19 (1906), Seiten 371-381, Zur Theorie der Brownschen Bewegung
[7] Bohren CF, Huffman DR. Absorption and scattering by a sphere. In: Bohren CF, Huffman DR, editors. Absorption and scattering of light by small particles. Weinheim: Wiley-VCH Verlag GmbH; 2007. pp. 83–129.